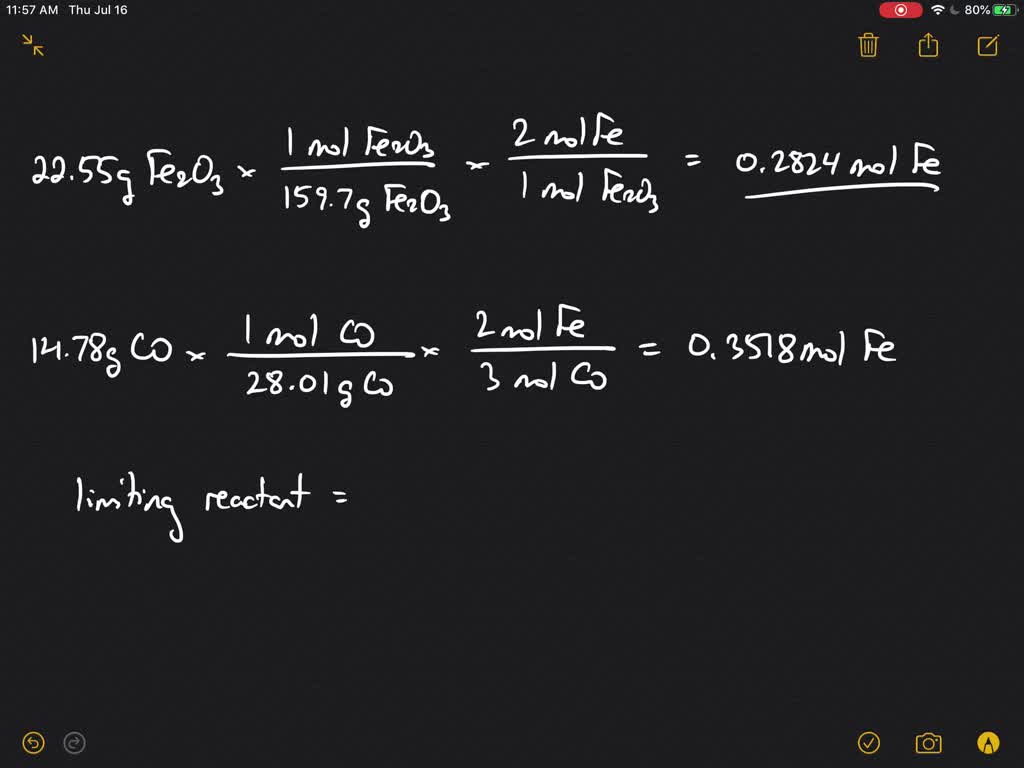
Iron Iii Oxide + Carbon Monoxide . redox reaction of iron iii oxide and carbon monoxide. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. This will melt and collect at the bottom of the furnace, where. The carbon monoxide in the blast furnace. To balance fe2o3 + co = fe + co2 you'll. the oxygen in the air reacts with the coke in the charge to form carbon monoxide. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (iii) oxide reacts with carbon monoxide according to the equation: Balancing redox reaction by oxidation number method:. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide as well as by reacting iron. here are the equations for the reaction.
from www.numerade.com
the oxygen in the air reacts with the coke in the charge to form carbon monoxide. To balance fe2o3 + co = fe + co2 you'll. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide as well as by reacting iron. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. The carbon monoxide in the blast furnace. redox reaction of iron iii oxide and carbon monoxide. iron (iii) oxide reacts with carbon monoxide according to the equation: Balancing redox reaction by oxidation number method:. here are the equations for the reaction.
SOLVEDIron(III) oxide reacts with carbon monoxide according to the
Iron Iii Oxide + Carbon Monoxide carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. Balancing redox reaction by oxidation number method:. iron (iii) oxide reacts with carbon monoxide according to the equation: iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide as well as by reacting iron. here are the equations for the reaction. This will melt and collect at the bottom of the furnace, where. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. To balance fe2o3 + co = fe + co2 you'll. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. The carbon monoxide in the blast furnace. redox reaction of iron iii oxide and carbon monoxide. the oxygen in the air reacts with the coke in the charge to form carbon monoxide.
From www.chegg.com
Solved Iron (III) oxide reacts with carbon monoxide to Iron Iii Oxide + Carbon Monoxide here are the equations for the reaction. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. To balance fe2o3 + co = fe + co2 you'll. redox reaction of iron iii oxide and carbon monoxide. Web. Iron Iii Oxide + Carbon Monoxide.
From www.slideserve.com
PPT What is the difference between a chemical reaction and physical Iron Iii Oxide + Carbon Monoxide here are the equations for the reaction. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. the oxygen in the air reacts with the coke in the charge to form carbon monoxide. iron (iii) oxide reacts with carbon monoxide according to the equation: redox reaction of iron iii oxide and. Iron Iii Oxide + Carbon Monoxide.
From www.numerade.com
Iron(III) oxide reacts with carbon monoxide according to the equation Iron Iii Oxide + Carbon Monoxide iron (iii) oxide reacts with carbon monoxide according to the equation: here are the equations for the reaction. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. redox reaction of iron iii oxide and carbon monoxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction. Iron Iii Oxide + Carbon Monoxide.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon monoxide to produce iron Iron Iii Oxide + Carbon Monoxide To balance fe2o3 + co = fe + co2 you'll. This will melt and collect at the bottom of the furnace, where. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. The carbon monoxide in the blast furnace. here are the equations for the reaction. the oxygen in the air reacts with. Iron Iii Oxide + Carbon Monoxide.
From www.slideshare.net
Chemical Reactions Iron Iii Oxide + Carbon Monoxide carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. iron (iii) oxide reacts with carbon monoxide according to the equation: iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Balancing redox reaction by oxidation number method:. here are the equations for the reaction. redox reaction of. Iron Iii Oxide + Carbon Monoxide.
From solvedlib.com
Iron(III) oxide reacts with caibon monoxide produce i… SolvedLib Iron Iii Oxide + Carbon Monoxide carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. Balancing redox reaction by oxidation number method:. the oxygen in the air reacts with the coke in the charge to form carbon monoxide. here are the equations for the reaction. iron (iii) oxide reacts with carbon monoxide according to the equation: Web. Iron Iii Oxide + Carbon Monoxide.
From www.coursehero.com
[Solved] 14) Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide + Carbon Monoxide carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. the oxygen in the air reacts with the coke in the charge to form carbon monoxide. This will melt and collect at the bottom of the furnace, where. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon. Iron Iii Oxide + Carbon Monoxide.
From www.chegg.com
Solved ? iron (III) oxide + ? carbon monoxide iron (II) Iron Iii Oxide + Carbon Monoxide Balancing redox reaction by oxidation number method:. This will melt and collect at the bottom of the furnace, where. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide as well as by reacting iron. Fe2o3 (s) + 3. Iron Iii Oxide + Carbon Monoxide.
From www.slideserve.com
PPT 29 When 84.8 g of iron (III) oxide reacts with excess of carbon Iron Iii Oxide + Carbon Monoxide iron (iii) oxide reacts with carbon monoxide according to the equation: the oxygen in the air reacts with the coke in the charge to form carbon monoxide. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide. Iron Iii Oxide + Carbon Monoxide.
From askfilo.com
WORKED EXAMPLE 6 Iron(III) oxide reacts with carbon monoxide to form iron.. Iron Iii Oxide + Carbon Monoxide the oxygen in the air reacts with the coke in the charge to form carbon monoxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. here are the equations for the reaction. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. To balance fe2o3 +. Iron Iii Oxide + Carbon Monoxide.
From www.chegg.com
Solved 3. Iron(III) oxide reacts with carbon monoxide to Iron Iii Oxide + Carbon Monoxide Balancing redox reaction by oxidation number method:. here are the equations for the reaction. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide as well as by reacting iron. carbon monoxide reduces the iron. Iron Iii Oxide + Carbon Monoxide.
From www.numerade.com
Iron(III) oxide reacts with carbon monoxide according to the equation Iron Iii Oxide + Carbon Monoxide here are the equations for the reaction. the oxygen in the air reacts with the coke in the charge to form carbon monoxide. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. iron (ii, iii) oxide can be formed from the reaction between iron(iii) oxide and carbon monoxide as well as. Iron Iii Oxide + Carbon Monoxide.
From www.chegg.com
Solved Iron (III) oxide reacts with carbon monoxide Iron Iii Oxide + Carbon Monoxide the oxygen in the air reacts with the coke in the charge to form carbon monoxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. The carbon monoxide in the blast furnace. iron (ii, iii) oxide can. Iron Iii Oxide + Carbon Monoxide.
From www.numerade.com
SOLVED47. Iron(III) oxide reacts with carbon monoxide equation Iron Iii Oxide + Carbon Monoxide the oxygen in the air reacts with the coke in the charge to form carbon monoxide. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (iii) oxide reacts with carbon monoxide according to the equation: Web. Iron Iii Oxide + Carbon Monoxide.
From slideplayer.com
(D) OXIDISING AND REDUCING AGENTS ppt download Iron Iii Oxide + Carbon Monoxide the oxygen in the air reacts with the coke in the charge to form carbon monoxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. This will melt and collect at the bottom of the furnace, where. To balance fe2o3 + co = fe + co2 you'll. The carbon monoxide in the. Iron Iii Oxide + Carbon Monoxide.
From www.coursehero.com
[Solved] Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide + Carbon Monoxide redox reaction of iron iii oxide and carbon monoxide. here are the equations for the reaction. the oxygen in the air reacts with the coke in the charge to form carbon monoxide. To balance fe2o3 + co = fe + co2 you'll. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron.. Iron Iii Oxide + Carbon Monoxide.
From www.slideserve.com
PPT 29 When 84.8 g of iron (III) oxide reacts with excess of carbon Iron Iii Oxide + Carbon Monoxide The carbon monoxide in the blast furnace. carbon monoxide reduces the iron (iii) oxide in the iron ore to form iron. iron (iii) oxide reacts with carbon monoxide according to the equation: the oxygen in the air reacts with the coke in the charge to form carbon monoxide. This will melt and collect at the bottom of. Iron Iii Oxide + Carbon Monoxide.
From www.coursehero.com
[Solved] 14) Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide + Carbon Monoxide This will melt and collect at the bottom of the furnace, where. To balance fe2o3 + co = fe + co2 you'll. iron (iii) oxide reacts with carbon monoxide according to the equation: The carbon monoxide in the blast furnace. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. carbon monoxide. Iron Iii Oxide + Carbon Monoxide.